There are two separate ways of looking at processes to a Chemist – Kinetic and Equilibrium. Equilibrium arguments predict where a process is going and what the end state will be. Kinetic arguments predict how fast it’s going to happen.
Using either alone may not be sufficient to characterize life. For example, glass remains a liquid. Equilibrium arguments, applied to all the windows of your house, predict they will slowly sag into pools of silicon. In fact, on older houses, you can start to see the ripples developing in the window panes. But functionally, kinetic arguments identify that it’s going to happen so slow that probably your great grand-daughter will be content looking out the same window with nary a thought about liquid windows.
I think most people know where they want to go in life. Or, where they should go in life. They know the equilibrium end state they want to reach. But almost always, the kinetics of the process are unsatisfying. Looking back over each 5 year interval, what I want to have happened just hasn’t happened fast enough.
Maybe a truly good thing happens when external circumstances force me to change faster than I’m comfortable with, but toward the equilibrium goal I know is right.

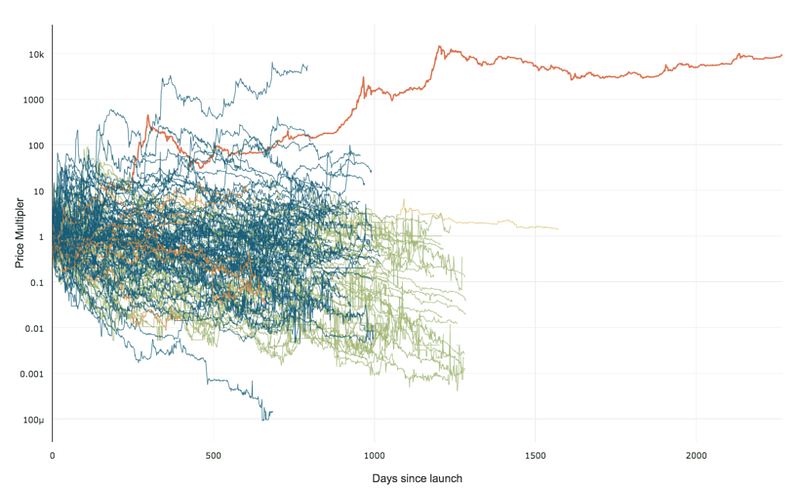 99% of ICOs Will Fail
99% of ICOs Will Fail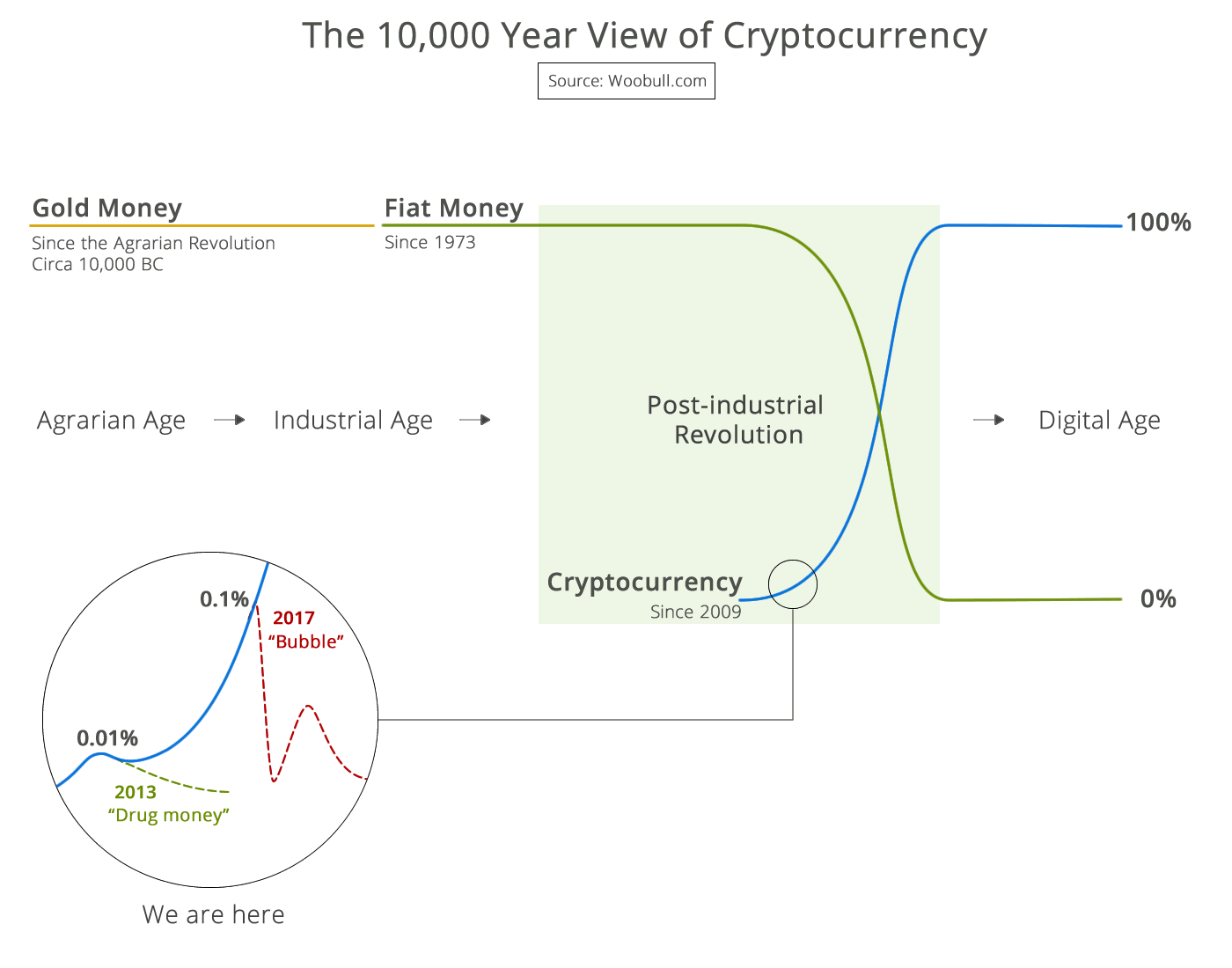 The 10,000 year view of cryptocurrency
The 10,000 year view of cryptocurrency